Background
The primary endpoint analysis of the GAIA trial showed superior progression-free survival (PFS) and undetectable MRD (uMRD) rates for venetoclax-obinutuzumab (GV) and GV + ibrutinib (GIV) compared to chemoimmunotherapy (CIT) (Eichhorst et al., NEJM 2023). With additional follow-up, outcomes of the venetoclax (ven)-containing arms were compared and NGS-based MRD results were analyzed.
Methods
The phase 3 GAIA trial compared 3 different time-limited ven-based combinations against CIT in fit, treatment-naïve patients (pts) with CLL without TP53 aberrations. Pts were randomized to CIT (FCR ≤65 years; BR >65 years), GV, GIV or ven-rituximab (RV). In addition to MRD by flow cytometry (FCM), exploratory MRD analyses were performed using the amplicon-based EuroClonality NGS assay. Reported p values have a descriptive character.
Results
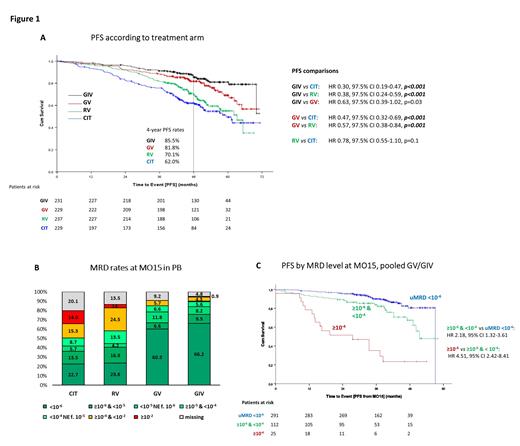
In total 926 pts were randomized (CIT: 229, RV: 237, GV: 229, GIV: 231). After a median observation time of 50.7 months (interquartile range 44.6-57.9), all pts are now off study treatment. PFS continued to be superior for GV and GIV compared to CIT (GV: median not reached [NR] vs 59.4 months; hazard ratio [HR] 0.47 [97.5% CI 0.32-0.69], p<0.001; GIV: NR vs 59.4 months, HR 0.30 [97.5% CI 0.19-0.47], p<0.001, Figure 1A). PFS with GV and GIV was also superior compared to RV (GV: NR vs 63.2 months; HR 0.57 [97.5% CI 0.38-0.84], p=0.001; GIV: NR vs 63.2 months, HR 0.38 [97.5% CI 0.24-0.59], p<0.001). PFS between GIV and GV was not significantly different (both NR, HR 0.63 [97.5% CI 0.39-1.02], p>0.025), however, GIV was associated with longer PFS compared to GV in pts with unmutated IGHV (HR 0.58 [95% CI 0.36-0.94]) but not in pts with mutated IGHV (HR 0.87 [95% CI 0.33-2.31]). Estimated 4-year PFS rates were 62.0% (CIT), 70.1% (RV), 81.8% (GV) and 85.5% (GIV). The estimated 4-year rates for time to next treatment were 77.2% (CIT), 86.2% (RV), 90.4% (GV) and 96.0% (GIV). Of the 111 pts with subsequent therapies for CLL-type progression (excluding 12 pts with treatment for Richter's transformation as second line), 60 (54.1%) received BTKi-based therapies, 30 (27.0%) ven-based treatments, 12 (10.8%) ven + BTKi and 5 (5.4%) CIT as second-line treatments. No differences in overall survival were observed between the treatment arms (4-year OS rates, CIT 93.5%; RV 96.2%; GV 95.1%; GIV 95.0%).
In a multivariate analysis, unmutated IGHV (HR 2.86 [95% CI 1.64-5.01], p<0.001) and bulky disease (any lymph node ≥ 5 cm, HR 1.73 [95% CI 1.11-2.69], p=0.016) were independently associated with shorter PFS in the pooled GV/GIV arms.
NGS-based MRD data in PB was available for 816 pts at month 15. Of these, 22.7% (52 pts, CIT), 23.6% (56 pts, RV), 60.3% (138 pts, GV) and 66.2% (153 pts, GIV) achieved uMRD <10 -6 (uMRD6, Figure 1B). In all treatment arms, PFS was shorter in pts with MRD ≥10 -6 compared to those with uMRD6 (CIT: HR 9.98 [95% CI 3.64-27.38], RV: HR 6.57 [95% CI 2.72-16.77], GV: HR 3.93 [95% CI 2.18-7.09], GIV: HR 2.10 [95% CI 1.03-4.28]). Pts who achieved uMRD below the conventional cut-off of 10 -4 by FCM but still had low levels of detectable MRD (≥10 -6 & <10 -4) by NGS had shorter PFS than pts achieving uMRD6 in the pooled GV/GIV arms (HR 2.18 [95% CI 1.32-3.61], Figure 1C). A similar correlation was seen with CIT (HR 4.49 [95% CI 1.53-13.14]) and RV (HR 3.40 [95% CI 1.29-8.98]). In pts with uMRD6 at MO15, clinical response (partial/complete response) did not influence PFS.
Grade ≥3 infections were highest in GIV and CIT (CIT: 45 pts [20.8%], RV: 27 [11.4%], GV: 34 [14.9%], GIV: 51 [22.1%]) and cardiac disorders most frequent with GIV (CIT: 14 pts [6.5%], RV: 19 [8.0%], GV: 18 [7.9%], GIV: 41 [17.7%]). Fatal adverse events occurred in 16 (7.4%, CIT), 8 (3.4%, RV), 9 (3.9%, GV) and 11 (4.8%, GIV) pts. The rate of second primary malignancies was higher with CIT (4.19/1000 patient-months) compared to RV (2.34), GV (2.39) and GIV (2.88). When excluding non-melanoma skin cancer, the incidence rates were 2.21 (CIT) 1.21 (RV), 1.16 (GV) and 2.36 (GIV).
Conclusions
With more than 4 years of follow-up, GV and GIV show superior PFS compared to CIT and RV. Pts with unmutated IGHV have longer PFS with GIV compared to GV. A majority of pts treated with time-limited GV or GIV (60.3% and 66.2%) achieves uMRD6 at MO15. NGS-based MRD assessment identifies pts with very long PFS and appears to improve prognostication in pts with uMRD <10 -4 by conventional FCM. Unmutated IGHV and bulky disease were independently associated with shorter PFS in pooled GV/GIV.
OffLabel Disclosure:
Fürstenau:BeiGene: Research Funding; AstraZeneca: Research Funding; Janssen: Research Funding; Roche: Research Funding; Abbvie: Honoraria, Research Funding. Ritgen:Janssen: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Other: travel support; Abbvie: Consultancy, Research Funding. Von Tresckow:AstraZeneca: Consultancy, Honoraria, Other, Speakers Bureau; Roche: Consultancy, Honoraria, Other, Speakers Bureau; Abbvie: Consultancy, Honoraria, Other: travel grant, Speakers Bureau; Janssen: Consultancy, Honoraria, Other, Speakers Bureau. Tadmor:janssen,: Research Funding; Abbvie, ROCHE, Janssen, AstraZeneca, takeda: Consultancy, Honoraria. Juliusson:AbbVie: Honoraria; Jazz: Honoraria; Servier: Honoraria; Laboratoire Delbert: Other: Research cooperation; Novartis: Honoraria. Janssens:Novartis: Speakers Bureau; Eli-Lilly: Speakers Bureau; Amgen: Speakers Bureau; Gilead: Consultancy; Abbvie: Consultancy; Roche: Consultancy; Takeda: Consultancy, Speakers Bureau; Sanofi: Speakers Bureau; Janssen-Cilag: Consultancy, Speakers Bureau; Beigene: Consultancy, Speakers Bureau; MSD: Consultancy; Argenx: Consultancy; AstraZeneca: Consultancy, Speakers Bureau. Levin:Abbvie: Honoraria; Janssen: Honoraria; Roche: Honoraria. Schneider:Abbvie: Honoraria, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; BeiGene: Other: travel support; Jannsen Cilag: Consultancy. Rossi:AbbVie, AstraZeneca, Gilead, BeiGene, BMS, Janssen, Lilly, Kyte: Honoraria, Research Funding. Frederiksen:Novartis: Research Funding; Sanofi: Research Funding; Alexion: Research Funding; Gilead: Research Funding; AbbVie: Research Funding; Janssen Pharmaceuticals: Research Funding. Kutsch:Celgene: Other: travel support; Gilead: Honoraria, Other: travel support, Research Funding; AbbVie: Honoraria, Other: travel support; Roche: Honoraria; BMS: Honoraria; AstraZeneca: Honoraria, Research Funding; Janssen: Other: travel support. Dürig:Roche: Consultancy, Honoraria, Other: travel support; Sanofi: Consultancy, Honoraria, Other: travel support; Beigene: Consultancy, Honoraria, Other: travel support; Celgene: Consultancy, Honoraria, Other: travel support; Janssen: Consultancy, Honoraria, Other: travel support; AstraZeneca: Consultancy, Honoraria, Other: travel support; Amgen: Consultancy, Honoraria, Other: travel support; Abbvie: Consultancy, Honoraria, Other: travel support. Böttcher:AstraZeneca: Honoraria, Speakers Bureau; Sanofi: Honoraria, Speakers Bureau; Janssen: Honoraria, Research Funding, Speakers Bureau; Abbvie: Honoraria, Speakers Bureau; Roche: Honoraria, Speakers Bureau. Simon:AstraZeneca: Research Funding; Lilly Pharma: Other: Travel support. Fink:Abbvie: Other: travel support; AstraZeneca: Consultancy, Honoraria, Research Funding. Fischer:Roche: Honoraria, Other: Travel Support; AstraZeneca: Consultancy; Abbvie: Honoraria, Other: TRavel support. Kreuzer:Abbvie: Consultancy, Research Funding, Speakers Bureau; Janssen: Consultancy, Research Funding, Speakers Bureau; Roche: Consultancy, Research Funding, Speakers Bureau. Brüggemann:Incyte: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Regeneron: Research Funding; Pfizer: Speakers Bureau; Affimed: Research Funding; BD: Speakers Bureau; Janssen: Speakers Bureau. Tausch:Janssen-Cilag: Consultancy, Honoraria, Other: travel support, Speakers Bureau; Abbvie: Consultancy, Honoraria, Other: Travel Support, Research Funding, Speakers Bureau; Roche: Consultancy, Honoraria, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Other: travel support, Speakers Bureau; BeiGene: Consultancy, Other: Travel support, Speakers Bureau. Stilgenbauer:Amgen: Consultancy, Honoraria, Other: travel support, Research Funding; Abbvie: Consultancy, Honoraria, Other: travel support, Research Funding; Celgene: Consultancy, Honoraria, Other: travel support, Research Funding; Gilead: Consultancy, Honoraria, Other: travel support, Research Funding; GSK: Consultancy, Honoraria, Other: travel support, Research Funding; Roche: Consultancy, Honoraria, Other: travel support, Research Funding; Janssen: Consultancy, Honoraria, Other: travel support, Research Funding; Novartis: Consultancy, Honoraria, Other: travel support, Research Funding; Sunesis: Consultancy, Honoraria, Other: travel support, Research Funding; AstraZeneca: Consultancy, Honoraria, Other: travel support, Research Funding. Hallek:AstraZeneca: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding. Kater:LAVA: Consultancy, Honoraria, Research Funding; Astra Zeneca: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Genentech, Inc.: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding. Niemann:Carsten Niemann has received research funding and/or consultancy fees from AstraZeneca, Janssen, AbbVie, Beigene, Genmab, CSL Behring, Octapharma, Takeda, and Novo Nordisk Foundation.: Consultancy, Research Funding. Eichhorst:AstraZeneca: Consultancy, Honoraria, Research Funding, Speakers Bureau; BeiGene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Gilead: Consultancy, Research Funding; Janssen: Consultancy, Research Funding, Speakers Bureau; Lilly: Consultancy, Speakers Bureau; MSD: Consultancy, Honoraria, Speakers Bureau; F. Hoffmann-La Roche Ltd: Honoraria, Research Funding, Speakers Bureau; Abbvie: Consultancy, Honoraria, Research Funding, Speakers Bureau.
The triple combination of venetoclax, ibrutinib and obinutuzumab is not approved for the treatment of CLL

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal